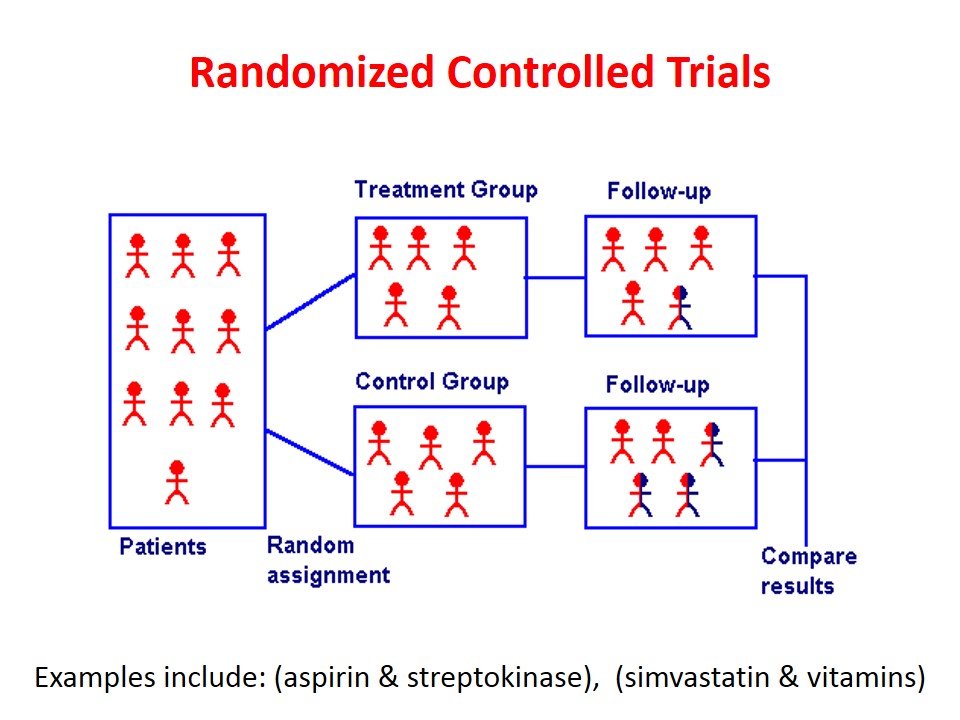
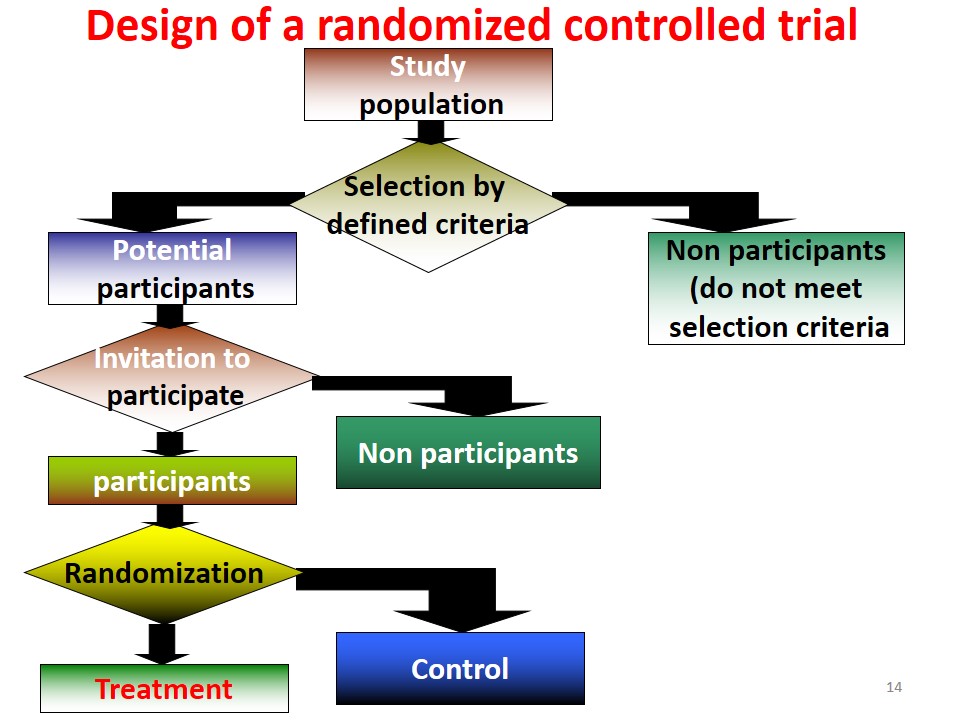
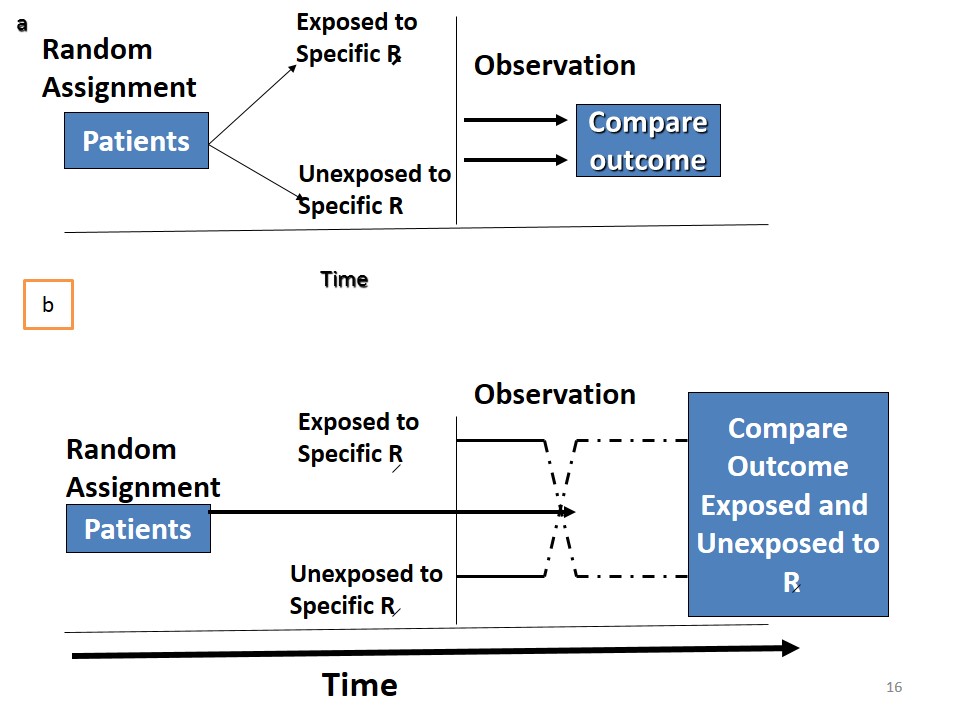
An epidemiological experiment in which subjects in a population are randomly allocated into groups, usually called study and control groups to receive and not receive an experimental preventive or therapeutic procedure, maneuver, or intervention is known as randomized controlled trial. John M.Last, 2001
This is the best method of evaluation, basic steps are:
1. Drawing up a Protocol
Drawing up a protocol includes defining:
• Objectives
• Questions to be asked
• Criteria for selection
• Size of sample
• Allocation in study & control groups
• Treatment to be given
• Standardization of Procedures
2. Selecting Reference & Experimental or Study Population
Reference Population: It is the population to which the finding of trial is to be applied.
Study Population: Actual population that participates in the study and is derived from the reference population.
3. Randomization
Each person in reference population must have equal chance of being included in study or control group
4. Manipulation
Deliberate application or withdrawal or reduction of suspected causal factor (vaccine, dietary component, habit).
5. Follow Up
Examination of the experimental and control groups at a defined interval of time.
6. Assessment
Assessment of out comes in terms of
• Positive result -Benefits
• Negative result -Side effects
Both are compared in both groups
Designs Used in Experimental Studies
1. Parallel Design
2. Cross Over Design
Advantages of Experimental Studies
• Exposure is under the control of investigator
• Randomization
• Blinding eliminates bias
• Control on time span
• Confounding factors can be controlled
• Best method to study causal relationship
• We can confirm or refute etiological hypothesis on evidence.
• Evaluate effectiveness and efficiency of health services
Disadvantages of Experimental Studies
• Subject exclusion may limit ability to generalize findings to other patients.
• A long period of time is often required to reach a conclusion.
• A large number of participants may be required.
• Financial costs are typically high.
• Ethical concerns may arise.
• Subjects may not comply with treatment assignments.
• Exposure or treatment alternatives should be acceptable to both groups
Selected Concepts
1. Control group
2. Randomization
3. Admissibility criteria
4. Outcome ascertainment
5. Ethics
All except #2 apply to observational designs as well
Control Group
The effects of an exposure can only be judged in comparison to what would happen in its absence. The control group provides this comparison
Illustration: “MRFIT”
MRFIT stands for ‘Multiple Risk Factor Intervention Trial’
Exposure was health education vs. no special intervention, outcome was considered to be CVD. The treatment group experienced dramatic declines in CVD. But so did the control group. CVD rates were declining in all groups in the 1970s. Thus the effect of the intervention was negligible.
Effects from inert interventions
Placebo effect
Improvement associated with inert interventions. Placebo effects are a scientific mystery
Hawthorne Effect
Subjects improve an aspect of their behavior being experimentally measured simply in response to the fact that they are being studied, not in response to any particular experimental manipulation
Randomization
Randomization works by balancing extraneous determinants in the groups being compared, thus mitigating confounding.
How randomization works?
Suppose you want to determine whether a particular diet (the exposure) is associated with improved weight gain in lab animals (outcome).
Randomization encourages equal numbers of fast-growing rats in each group.
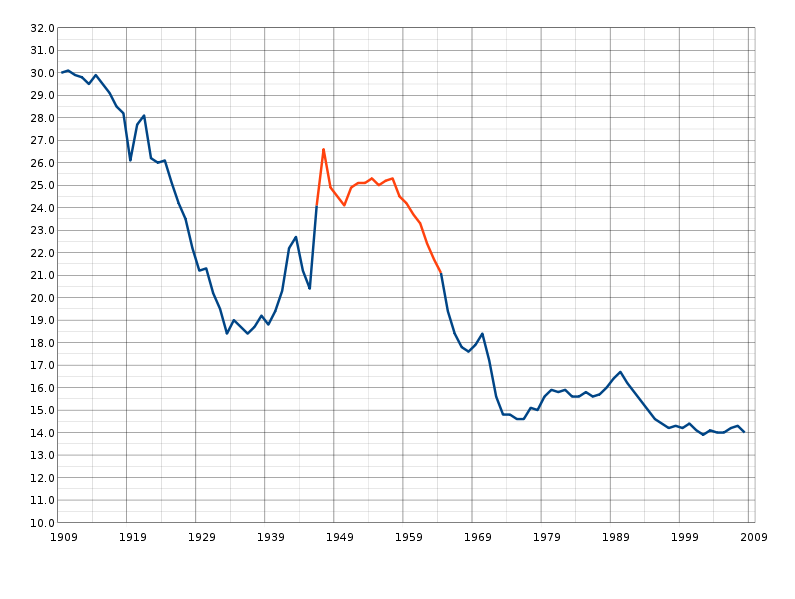
Polio Field Trial (1954)
Polio rates (per 100,000) were found to be 69 among placebo, 46 among refusers and 28 among vaccinated individuals.
Note: Had refusers been used as the control group, the effects of the intervention would have been underrated.
(Am J Pub Health, 1957, 47: 283-7)
Admissibility Criteria
Restrict participants to those with uniform characteristics. This too mitigates confounding
Example: Excluding smokers from a study base would prevent confounding from smoking
Outcome Ascertainment
Outcome ascertainment must be valid. Without valid outcome ascertainment, we have GIGO (garbage in, garbage out)
Ethics
Ethics include:
• Respect for individuals
• Beneficence
• Justice
• oversight
• Informed consent
Unique Problems of Intervention Studies
Ethics
Sufficient doubts to withhold from half the population and sufficient believes to expose half the population, thus require high scientific standards
Feasibility
Widespread adaption of measures by community is often difficult thus there are problems of finding sufficiently large eligible sample size
Costs
Expensive
Summary
Gold standard in epidemiological research
• Makes study groups comparable
– Random allocation
– Sufficient sample size
• Unique problems of ethics, feasibility and costs
• Ensure transparency of all trials
 howMed Know Yourself
howMed Know Yourself