Amphotericin B
Source
- Antibiotic obtained from Streptomyces nodosus.
- Amphotericin A –no therapeutic application
- Amphotericin B –broader activity, antifungal
Chemistry
7 Polyene macrolide-O-Mycosamine
Polyene is a term used for agents consisting of large number of double bonds. Macrolides are molecules having more than 12 atoms.
General Properties
- Poorly water soluble
- Mostly available as a suspension with sodium desoxycholate
- Amphoteric molecule –amphipathic character in addition. Both water and lipid soluble components
- Stable at extremes of pH
- Latest prepatation besides colloidal and sodium desoxycholate is Liposomal amphotericin B to decrease renal toxicity of amphotericin B.
Hydroxyl groups are capable of producing mechanism of action as antifungal.
Mechanism of Action
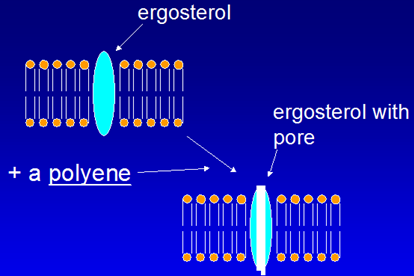
Sterol component in fungal cell membrane is ergot instead of cholesterol.
Has amphipathic character, both lipophilic (due to choline) and hydrophilic components.
When administered:
- Preferential binding to ERGOSTEROL, choline part binds
- Pores or channels are formed in membranes of sensitive fungi (lipophilic part)
- Cell is unable to maintain internal environment, expulsion of intracellular ions occur
- Fungicidal action
Resistance
Resistance occurs because different mechanisms are established:
- Some defect in binding site of ergosterol for amphotericin B
- Less affinity for amphotericin B
- Less synthesis of ergosterol due to which resistance occurs.
Some binding of amphotericin B with human tissues is responsible for side effects.
Spectrum of Activity (The broadest spectrum)
Broad spectrum antifungal agent mainly effective against most fungi causing mycotic infection including:
- Candida albicans
- Cryptococcus neoformans
- Histoplasma capsulatum
- Blastomyces dermatitidis
- Coccidioides immitis
- Aspergillus fumigatus
Pharmacokinetics
- Oral absorption poor thus not given orally. Locally applied for irrigation of bladder, gut.
- I/V 0.6mg/kg/day, with this dose blood levels are maintained at 0.3-1 mcg/ml.
- Protein binding 90%
- Wide distribution (CNS 2-3%) fungal meningitis?
- Thus if required intrathecally administered for achieving higher levels, having some neurotoxic manifestations (some neurotoxic manifestations).
- Excretion in urine and bile. Traces in urine are found for several days due to wide distribution and high plasma protein binding.
- t1/2 15 days
- Liposomal amphotericin B –newer amphotericin B
AmBisome 3-5mg/kg/d
Amphotec 5mg/kg/d
Abelcet 5mg/kg/d
- Older preparations include colloidal suspensions and sodium desoxychalate.
- In order to decrease toxicity liposomal preparations are made. Amphotericin B attaches to lipid carriers. Liposomes act as a reservoir of amphotericin B. Most of it is within liposomal preparations, so its attachment to renal cells decreases renal toxicity.
- Enzymes known as lipases cause release of bond from amphotericin B, increasing their availability for physical effects.
- Free form is made available producing more efficacy.
- These drugs are highly expensive and not cost-effective. Used for renal mainifestations.
Toxicity
Immediate
Seen when given I/V. Manifested by:
- Fever,
- chills,
- muscle pain,
- headache
- vomiting,
- hypotension
Treatment:
- Test dose:- 1mg amphotericin B I/V in 20 ml of 5% dextrose
- Daily doses decreased if fear for immediate effects.
- Dose has to be administered slowly
- If such manifestations, then premedication with anitpyretics and antihistaminics or corticosteroids.
Late
1. Renal damage (reversible –deviation of RFTs and decreased GFR)
(Irreversible >4g total dose)
2. Renal Tubular Acidosis –accumulation effect beyond 4g.
3. Severe hypokalemia. Treated by calcium supplements,
4. Hypomagnesemia (K+ Mg++ loss)
5. Abnormal liver function test
6. Anemia of renal origin –reversible normocytic normochromic
7. Loss of Erythropoietin formation
Due to renal damage. When therapy is stopped, anemia is corrected.
8. Cerebral damage –Seizures
When given intrathecally for fungal meningitis, producing neurotoxic effects including headache and seizures.
Therapeutic uses
1. Induction Therapy
Broad spectrum, mainly used for systemic fungal infections. Initially introduced as induction therapy for fungal infections, later maintenance with newer azoles.
- Fungal pneumonia
- Meningitis
- Immunocompromised;
- neutropenic patients
- Fungal sepsis
Dose 0.5-1 mg/kg/d (Total dose 1-2g)
2. Locally –empirical therapy of neutropoenia
- Mycotic corneal ulcer,
- keratitis,
- Fungal arthritis (injected directly into joints)
- Candiduria
3. Intrathecal
Care is taken to avoid neurotoxicity.
Griseofulvin
Systemic drug used for local infection and not systemic. Uses are declining, grouped with antibiotic group. Obtained from same source as penicillin.
Mechanism of action
- When given orally, binds to microtubules comprising the spindles and inhibits mitosis.
- Incorporates into affected keratin –fungistatic
- Inhibit nucleic acid synthesis
- Taken with fatty meal as bioavailability is increased.
- Microfine dosage form or micro crystalline form
Dose: 1g/day dose
- Half life is 24 hours.
- For hair infections administered for 4-6 weeks.
- For nails infections administered for 4-6 months.
- For toe nail infections administered for 8-18 months.
Should be given for prolonged period so that growth of newer skin, hair are devoid of fungal infections.
Uses
Tinea infections of skin, hair and nail due to:
- Microsporam
- Trichophyton
- Epidermophyton
- Athletes foot
Both systemic drugs used for topical infections.
Cure rate high given for Weeks to Months
Nystatin
A polyene macrolide obtained from penicillin.
Discovered in New York state lab so named.
Mechanism of Action
- It is a polyene macrolide ,similar in structure & mechanism to amphotericin B.
- Too toxic for systemic use.
- Used only topically but not recommended for oral use as has very bad taste and serious adverse effects.
- It is available as creams, ointment , suppositories & other preparations.
- Not significantly absorbed from skin, mucous membrane, GIT.
- Very less amount is absorbed systemically, toxicity chances are less
Uses
Topically for candidiasis
- Prevent or treat superficial candidiasis of mouth, esophagus, intestinal tract.
- Vaginal candidiasis
- Can be used in combination with antibacterial agents & corticosteroids
Continue Reading
Anti Fungal Drugs -Azoles, Echinocandins, Allylamines and Flucytosine
 howMed Know Yourself
howMed Know Yourself