Introduction
- Analgesia means insensibility to pain. Drugs used in management of pain are known as analgesics.
- Pain serves the useful function of alerting an individual that some component of physiological system has gone awry.
- Pain is most effectively treated by removal of underlying cause.
- Physician is often faced with the necessity for treating pain as a symptom
- Pain can be divided into acute & chronic
- Chronic pain can endure long after healing.
- Acute pain is directly related to a noxious stimulus
- Pain is due to the release of prostaglandins, substance P and bradykinin, which contribute to pain.
- Acute pain has two components:
- Phasic
- Tonic components
Phasic pain is produced by the presence of a noxious stimulus
It is superficial pain and involves skin Produces intense sharp sensation. It is transmitted through, fast conducting myelinated A-delta fibers
Tonic pain is evoked by tissue damage occurring in the skin or in the viscera, gradual onset than phasic pain, dull throbbing aching pain. It is conducted through the unmyelinated slow conducting C-fiber pathways.
- Drugs can alter pain experienced in several ways. Pain is sensation, perception and has emotional aspect as well.
- Pain is not a single entity; it is pain of the entire inflammatory process and one of the clinical signs of inflammation.
- Pain can be described as Acute or chronic and associated with malignant disease or chronic and not associated with malignant disease.
- The duration of acute pain is usually hours to days while the chronic pain can last for months to years and have associated problems of depression and anxiety.
- Drugs may act as analgesics by altering either sensational or emotional aspects of pain. Adjuvant drugs are also used like hypnotics, antidepressants to reduce anxiety and depression.
- Oral pain is associated with pulpitis, periodontitis, abscesses, temporomandibular disorders (TMDs) and masticatory muscle disorder. Dental procedure also can have pain.
- Pain inducing substances can be produced and released from cell membranes by trauma, (e.g. mechanical trauma to the soft tissues and bone during periodontal surgery), infection and allergenic reactions.
- The majority of dental pain is an acute response to inflammation, usually managed pharmacologically.
- For moderate pain that is not relieved by NSAIDs, opioid analgesics are the agents of choice. Opioids alter the patients perception of the pain in the brain.
- Combined use of a NSAIDs with an opioid produces a synergistic effect so that a lower dose of opioid can be used.
- Opioids alone are not prescribed for the management of acute dental pain because to high a dose would be needed. The adverse side effects would not be acceptable.
- Analgesics are divided as those
- Acting on the CNS i.e. Opioids.
- Acting peripherally – NSAIDs
Nociceptors: are specialized sensory nerve endings, which when stimulated may lead to the sensation of pain
Pain may be of visceral or somatic type:
Visceral pain arises from viscera is dull and less localized. It is treated by opioid analgesic
Somatic pain arises from musculoskeletal organs, skin and muscle, it is localized & sharp & is treated by NSAIDS
Opioids act at CNS and alter the patient’s perception. They are used in severe types of pain. They alter perception, intensity and have emotional effects as well. They are obtained from natural source, opium poppy.
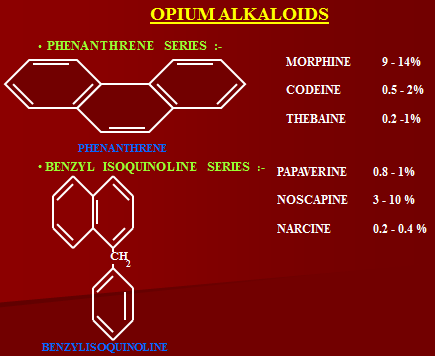
Opium has two series of alkaloids:
- Phenanthrene
- Benzyl isoquinaline
Opioid drugs mimic the actions of 03 peptide families in the brain, known as:
- Endorphins
- Enkephalins
- Dynorphins
The peptides along with non-opioids peptides are cleaved from the protein precursors
– Pro-opiomelanocortin (POMC)
– Proenkephalin
– Prodynorphins
Papaverine is a vasodilator
Noscapine is cough suppressant and does not has analgesic property.
Types of receptors
- Mu (µ)-most actions mediated
- Kappa
- Sigma
- Delta
- Epsilon
- Mu receptors
These are responsible for pharmacological actions of morphine.
- Respiratory depression, sedation is produced, slowed GI+ transit
- Modulation of hormone and neurotransmitter release.
- Supraspinal and spinal analgesia.
- Kappa receptors
- Produce supraspinal and spinal analgesia
- Contribute to psychomimetic action
- Slowed GI Transit time
- Delta receptors
- Supraspinal and spinal analgesia
- Modulation of hormone and neurotransmitter release.
ORI-I
Orphanin opioid-receptor like subtype-I, which is an endogenous receptor.
Mechanism of action
- Opioid receptors are located on primary afferents and spinal cord pain transmission neurons (Ascending pathways).
- On neurons in the mid brain and medulla (descending pathway) that function in pain modulation.
- Opioid receptors involved in altering reactivity to pain are located on neurons in the basal ganglia, the hypothalamus, limbic structures and the cerebral cortex.
- Opioid analgesic inhibit synaptic activity through direct activation of opioid receptors and partly through release of the endogenous opioid peptides.
- Opioid receptor are coupled to their effectors by G proteins and activate phospholipase C or inhibit adenyl cyclase
- At the postsynaptic level, activation of these receptors can open K+ ion channels to cause membrane hyperpolarization (inhibitory postsynaptic potential)
- At the presynaptic level, opioid receptor activation can close voltage gated Ca++ ion channels to inhibit neurotransmitter release
- Presynaptic actions result in the inhibition of release of multiple neurotransmitter (Ach, NE, 5HT, glutamate & substance-P)
Chemical classification
Phenanthrene Alkaloids of Opium
- Morphine,
- Codeine
Semisynthetic Alkaloids of Opium
- Hydrocodone,
- Oxycodone,
- Buprenorphine
Totally Synthetic
Phenanthrene Derivatives
- Tramadol (dependence, tolerance not seen as with others)
Phenylpiperidines
- Meperidine (Pethidine)
- Fentanyl,
- Sufentanil,
- Alfentanil
- Remifentanil
Morphinan Derivatives
- Levorphanol,
- Butorphanol
Phenylheptylamine Derivatives
- Methadone,
- Levomethadyl Acetate,
- Propoxyphene
Benzoprophan Derivatives,
Opioid Agonists
- Morphine
- Codeine
- Hydrocodone
- Oxycodone
- Levorphanol
- Meperidine
- Methadone
- Levomethadyl Acetate
- Fentanyl
- Sufentanil
- Alfentanil
- Remifentanil
Partial Agonist (Mixed Agonist/Antagonist)
- Buprenorphine
- Butorphanol
- Pentazocine
Antagonists
- Naloxone (parenteral)
- Naltrexone (oral, parenteral)
- Nalmefene
Pharmacological Actions Of Opioids
Morphine is the prototype agonist.
Predominantly act on CNS and GIT. Alter pain perception in CNS.
1. CNS
Both depression and stimulation of CNS occurs.
– Analgesia/sedation (improve perception to pain, pain not felt both at spinal and supraspinal level, reduce substance P from afferent neurons
– Euphoria with rapid I/V injection
– Respiratory depression –decrease rate and depth of tidal volume, hypoxic drive is depressed
– Depression of Cough reflex
Calming effect, mental clouding, unable to concentrate
Vasomotor center is depressed, leading to bradycardia
Usual dose is 10 mg subcutaneous or I/M.
I/V 3-4 mg
Oral absorption is erratic.
2. Eye
Miosis by a direct action in the brain nucleus of the oculomotor nerve (edinger-westphal). Classic pinpoint pupil is important diagnostically.
Morphine if applied locally produces no mitotic effect. No tolerance develops to mitotic effect of opioids.
3. Emesis
Morphine directly stimulates CTZ in the area postrema that causes vomiting..
4. CVS
– Morphine has no effect at therapeutic doses. Large doses cause hypotension bradycardia.(because of histamine and vagal stimulation)
– Because of respiratory depression and Co2 retention, cerebral vessels dilate and increase (CSF) pressure.
Not administered to those with head injury because of CO2 retention.
5. GIT
Morphine increases the resting tone of the smooth muscle of the entire GI tract.
This result in a decrease in the movement of stomach and intestinal contents increase tone of the anal sphincter.
Spasm and constipation
Spasm of the smooth muscle of the biliary tract increases pressure, which may lead to biliary colic. Other drugs are used in biliary colic due to increased tone of sphincter of Odii and increase in pressure
6. Genitourinary System
In elderly, increase in tone and spasm of the smooth muscle. This can lead to urinary retention.
Hyperglycemia is due to central sympathetic stimulation in increased doses.
7. Histamine Release
Morphine releases histamine from mast-cell causing urticaria, sweating and vasodilatation. May lead to bronchoconstriction as well.
8. Temperature
µ receptor agonists act on anterior hypothalamus, producing hyperthermia.
Action on kappa receptors causes hypothermia.
In over dosage, there is always subnormal temperature, due to:
- Increased sweating
- Kappa activation
Clinical Uses
1. Analgesia
Morphine, Fentanyl
In cardiac and asthmatic patients.
- Cancer pains
- Pain of MI
- Obstetric labour
- Renal and biliary colic
- Post operative pain
2. Cough suppression
Codeine, Dextromethorphan
3. Treatment of diarrhoea
Diphenoxylate, loperamide
Diphenoxylate is given with atropine, not as anti-spasmodic, but in small quantity to prevent abuse liability.
However, opioid analgesics are not used in bacterial diarrhoea, because diphenoxylate decrease GI mobility, stopping diarrhoea but increasing the absorption of toxins.
Also not used in children, producing post diarrheal dilation ileus.
4. Management of acute pulmonary edema
Morphine given along with furosemides and oxygen.
Mechanism is:
- Anxiolytic effect –decreases perception of shortness of breath
- Decreases preload and after load, it directs pulmonary blood to periphery.
5. Anaesthesia
Morphine, Fentanyl (preanesthetic)
- Preanesthetic medication
- Intra op in combination with other aesthetic agents
- Mostly used in cardiovascular surgeries and those in which minimum CVS suppression is required
- As regional anaesthesia in epidural and subarachnoid spaces.
6. Shivering
Meperidine causes alpha 2 activation in CNS, decreasing shivering.
Opioid dependence
Main problem is development of tolerance and dependence. Tolerance does not develop in therapeutic dose, but only when withdrawn abruptly. Reason being adaptive changes at cellular levels.
Methadone is weak long acting agonist; chances of withdrawal symptoms are less.
Routes of Administration
- Rectal suppositories- Morphine, Hydromorphone
- Transdermal patch– Fentanyl
- Intranasal———— Butorphanol
- Buccal—————– Fentanyl citrate, Buprenorphine (mixed agonist and antagonist, cannot be antagonized by naloxone so not used)
Morphine and hydromorphine are given orally or parentally, rectal suppositories are also available.
Respiratory depression is the most serious complication of opioids, mediated through mu receptors, antagonized by naloxone 2-4 mg I/V.
Oral route is not preferred. Subcutaneous or I/V mostly used.
Adverse effects
- Behavioural restlessness, tremulousness
- Dysphoria when administered with no pain
- Muscle rigidity, convulsions
- Cortical areas/hippocampal stimulation leading to convulsions in over dosage
- Respiratory depression -emphysema
- Nausea and vomiting
- Increased intracranial pressure, leading to respiratory depression and CO2 retention
- Postural hypotension, bradycardia, vagal stimulation
- Constipation
- Urinary retention in elderly males due to actions on urethra
- Itching around the nose
- Idiosyncratic allergic reactions because of histamine release.
- Tolerance (physical, physiological) and dependence.
- Withdrawal syndrome
- Hypothermia in higher doses.
- Drug is avoided during pregnancy or labour. I/V or I/M may cause foetal distress, alternatives are used. Can be used intrathecally at labour, as there is no respiratory depression, constipation or dependence.
Avoided as routine analgesic. Immediate tolerance develops to CNS actions, some to bradycardia, but some actions do not develop tolerance:
- Miosis –route is different so no tolerance
- Constipation
- Convulsions due to cortical and hippocampal cells
- Some antagonist actions do not develop tolerance.
Dose has to be increased against tolerance.
Withdrawal is prevented by long acting opioid agonist.
Contraindications
- Use of pure agonist with weak partial agonist
- Use in patients with head injuries
- Use during pregnancy
- Use in patients with impaired hepatic or renal function
- Use in patients with impaired pulmonary function
- Use in patients with endocrine diseases like Addison’s disease & Hypothyroidism show exaggerated responses to opioids.
3 and 6 morphine glucuronide are the metabolites. 6 morphine glucuronide is the active metabolite, more active than parent drug. It undergoes enterohepatic circulation and is avoided in impaired hepatic and renal functions.
Pharmacokinetics
- Absorption of morphine from GIT is slow and erratic
- Significant first pass metabolism in the liver
- Rapidly distributed to all body tissues
- Morphine is least lipophilic, as compared with other opioids
- Only a small amount crosses blood brain barrier
- Metabolized to morphine-6-glucuronide and active metabolite is potent analgesic
- Excreted in urine and bile duration of action is 4-6 hrs.
- 3 morphine glucuronide is a neuroexcitator in the GABA glycinergic pathway. It does not act through the mu pathway. On prolonged use, it may lead to seizures. This does not occur in therapeutic doses.
- Increased sensitization to morphine occurs on continuous use, hypersensitivity is at spinal cord level.
- Codeine is another natural opioid agonist, on administration it is converted to morphine; itself has less affinity for mu receptors, so used as cough suppressant. It has least abuse liability and is non-addictive. But is often misused. Draw back in cough suppressant can lead to accumulation of secretions, resulting in obstruction of airways. Thus, atelectasis may occur.
Acute morphine poisoning
Signs and symptoms
- Coma
- Pinpoint pupil
- Respiratory depression (chyene stokes breathing)
- Bradycardia
- Hypothermia
- Decreased urine
- Decreased bowel sounds
- Pulmonary edema
- Cyanosis
Management
- Oxygen inhalation
- Intravenous fluid
- Decontamination (gastric lavage)
- Potassium permanganate administration, oxidizes the opioids
- Naloxone
- Steroids to counter histamine release
- Naltrexone
- Obscure the patient from withdrawal symptoms
 howMed Know Yourself
howMed Know Yourself