What is Bias?
An error in sampling or testing that systematically under- or over-represents one outcome (answer) over the other. Or a distortion in the perception of the effects of a treatment or in the measurement of difference between the effects of two treatments.
Bias will tend to mask the true strength of association.
Types of Biases
There are basically only three major types of biases.
1. Selection bias
2. Response bias
3. Information bias
All others are simply varieties of these three types
Selection Bias
Selection bias is caused by nonrandom sampling, so that a systematic difference is present between people selected for the study and people not selected for the study. It can be caused by convenient sampling, patient referral patterns, survival differences or loss to follow-up. This is an avoidable bias, and if not eliminated, can ruin the chances of acceptance or publication of the study.
Information (Measurement) Bias
A systematic difference between the measurements (or information) recorded in different study groups is known as information bias.
For example, in cohort studies, people with the risk factor may be tested more frequently and carefully than the control group. This is also called ‘surveillance’ bias or ‘diagnostic suspicion’ bias.
Interviewer Bias
An interviewer’s knowledge may influence the structure of questions and the manner of presentation, which may influence responses. Interviewer’s IQ may also influence in understanding the responses. Observers may have preconceived expectations of what they should find in an examination.
Recall Bias
A type of information bias, when people with a certain condition are more likely to remember exposure to the risk factor under study than the control group. It can occur easily in case-control or cross-sectional studies, but not in cohort studies (those with a particular outcome or exposure may remember events more clearly or amplify their recollections).
For example parents of children with cancer may ‘remember’ more information about details of risk factors and their exposure to them, than parents of control children with identical exposure rates.
Attrition Bias (Loss to follow-up)
Attrition is a reduction in the number of patients who remain in the study (patient drop out). This results in an attrition bias when the patients who drop-out of the study are systematically different from those who remain in and complete the study. It can occur in clinical trials and in cohort studies.
Admission Rate (Berkson’s) Bias
A selection bias that occurs when the hospital admission rate of controls and cases are different. It is likely to seriously affect odds ratio (OR) values in case-control studies, as controls are likely to be admitted less frequently than cases. Patients with two or more overlapping conditions are more likely to be admitted. Researchers may mistakenly assume an association between two or more conditions.
Prevalence / Incidence (Neyman’s) Bias
It occurs when a disease is characterized by early fatalities (death before diagnosis) or silent cases (no evidence of exposure at time of disease onset). It occurs whenever there is a ‘time gap’ between ‘exposure and subjects’ selection, so that the ‘worst’ cases have died out.
A cohort study begun before the disease will detect occurrences correctly. A case-control study begun later will only record the cases that have remained and did not die.
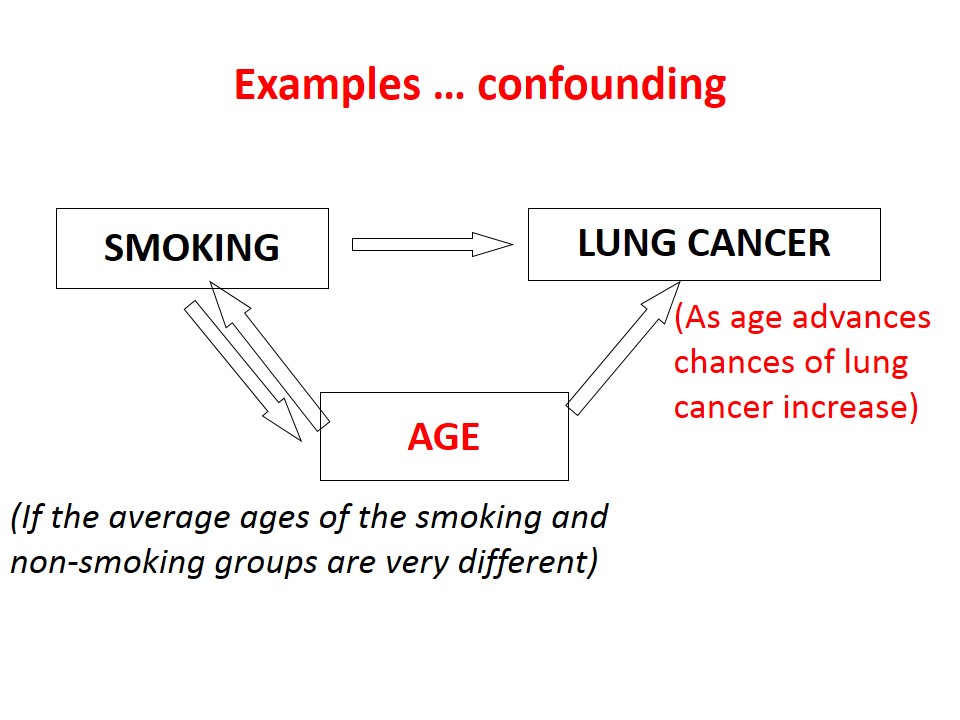
Confounding Bias
Confounding is the confusion or mixing of effects. A confounder is an extraneous variable that totally or partially accounts for the apparent effect of the study exposure on the outcome. It may even mask an underlying true association or reverse it. The distortion can be large and leads to over-estimation or under-estimation of an effect, it can even change the apparent direction of an effect.
Confounder must be:
• Risk factor among the unexposed (itself a determinant of disease)
• Associated with the exposure under study.
• Unequally distributed among the exposed and the unexposed groups.
Methods for controlling Selection Bias
During Study Design
1. Randomization
2. Restriction
3. Matching
During analysis
1. Stratification
2. Adjustment
a) Simple / standardization
b) Multiple / multivariate adjustment
c) Best case / worst case analysis
Randomization
In studies investigating the effects of therapy or other interventions, it is possible to reduce confounding by randomization. The randomization procedure randomly assigns patients to an experimental group or to a control group.
Randomization helps to prevent selection bias by the clinician (sometimes also referred to as ‘confounding by indication’).
Although randomization of large groups of patients will frequently result in a similar distribution of known and unknown confounders in the experimental and the control group, it is unlikely that this balance will be achieved for all patient characteristics. Though the balance may be incomplete, the randomization process does guarantee that any differences between the two groups are due to chance and not to the choice of the physician. Thus, although differences in potential confounders between the two groups may still exist after randomization, they are likely to be reduced as much as possible.
Examples of large randomized controlled trials in nephrology include the HEMO, ADEMEX and CREATE studies. The size of these RCTs helped the randomization process to be successful in producing a similar distribution of known confounders between the experimental groups.
A small RCT may fail to produce similar experimental groups. Investigators may then adjust for these confounders during data analysis.
Restriction
Subjects chosen for study are restricted to only those possessing a narrow range of characteristics, to equalize important extraneous factors.
Limitation: Generalizability is compromised; by excluding potential subjects, cohorts / groups selected may be unusual and not representative of most patients or people with condition.
Example
Study: Effect of age on prognosis of MI
Restriction: Male / White / Uncomplicated anterior wall MI
Thus important extraneous factors are controlled for sex / race / severity of disease but the limitation is that the results not generalizable tofemales, people of non-white community, those with complicated MI.
Matching
The process of making a study group and a comparison group comparable with respect to extraneous factors. (Last)
For each patient in one group there are one or more patients in the comparison group with same characteristics, except for the factor of interest. (Fletcher).
 howMed Know Yourself
howMed Know Yourself