Lipid Transport in Blood
} Lipids are not water soluble
} Lipids packed in protein
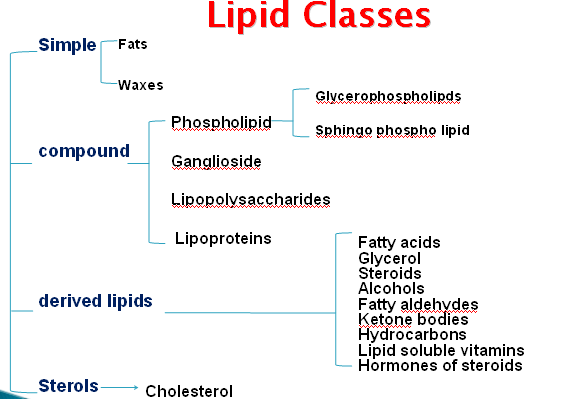
} Four classes of lipoproteins
◦ Chylomicrons
◦ VLDL
◦ LDL
◦ HDL
Lipid Digestion/Absorption
Five different phases:
¶ hydrolysis of triglycerides (TG) to free fatty acids (FFA) and monoacylglycerols
· solubilization of FFA and monoacylglycerols by detergents (bile acids) and transportation from the intestinal lumen toward the cell surface
¸ uptake of FFA and monoacylglycerols into the cell and resynthesis to triglyceride
Í packaging of TG’s into chylomicrons
Î exocytosis of chylomicrons into lymph
Enzymes Involved in Digestion of Lipids
} lingual lipase: provides a stable interface with aqueous environment of stomach
} pancreatic lipase: major enzyme affecting triglyceride hydrolysis
} colipase: protein anchoring lipase to the lipid
} lipid esterase: secreted by pancreas, acts on cholestrol esters, activated by bile
} phospholipases: cleave phospholipids, activated by trypsin
Repacking in the Liver:
} Lipid is repackaged in the liver to VLDL or very low density lipoprotein
} Lipoproteins are classified by density
} Lipoproteins transport lipid to the rest of the body
Lipid Transport
} Resynthesis of TAG and Cholesteryl ester
} Secretion from Enterocytes
} Use of Dietary Lipid
- Free fatty acids transported as complex with albumin in blood
- Glycerol converted to glycerol-3-phosphate, deliver to glycolysis pathway
Oxidation of Fatty Acid
β-Oxidation
α-Oxidation
ɷ-Oxidation
β-Oxidation
LCFA enter in cell and form CoA derivatives in cytosol
Enzyme: Fatty acyl CoA synthetase
Carnitine derivatives
LCFA Translocation
Ø Transfer acyl group from CoA to carnitine by palmitoyltransferase-I
Ø Acyl carnitine is transported into mitoch-matrix by carnitine-acylcarnitine translocase
Ø Carnitine-palmitoyltransferase-II transfer acyl gp to CoA and regenerate free carnitine
α- and ɷ-oxidation of fatty acids are specialized pathways
α -oxidation i.e., removal of one carbon at a time from the carboxyl end of the molecule has been detected in brain tissue. It does not generate CoA intermediates and does not generate high-energy phosphates.
ɷ-oxidation is a minor pathway and is brought about by cytochrome P450 in the endoplasmic reticulum. CH3 group is converted to a -CH2OH group that subsequently is oxidized to -COOH, thus forming a dicarboxylic acid. They subsequently undergo ß-oxidation and are excreted in the urine.
Unsaturated Fatty Acids
} Unsaturated fatty acids must be saturated before beta-oxidation
◦ Isomerase converts cis to trans and moves double bond to the 2 position
◦ In polyunsaturated: need reductase
Add H’s to second double bond
Odd Chain Fatty Acids
} Minor species, odd chains made by microbes, degradation of AA’s
} B-oxidation occurs to end:
◦ Left with 3 carbon + CoA
} Vitamin B12 cobalamin co-enzyme
◦ Catalyzes conversion of propionyl CoA (3 C) to succinyl-CoA (4 C)
TCA cycle intermediate
KETOGENESIS
It occurs when there is a high rate of fatty acid oxidation in the liver.
Acetoacetic acid
Hydroxybutyric acid
Acetone
These three substances are collectively known as the ketone bodies (also called acetone bodies or acetone). Enzymes responsible for ketone bodies formation are associated with mitochondria.
The main factors which control Ketogenesis in the liver
- Availability of the substrate (Long Chain Fatty Acids) : from increased production by lipolysis with increased delivery of FA to the liver.
- The level of Malonyl Co A in the liver, with its influence to inhibit the Carnitine Palmitoyl Transferase I (CPT I)
- The Glucagon / Insulin Ratio : a high ratio increases lipolysis and activation of oxidative ketogenesis , a low ratio counteracts ketogenisis
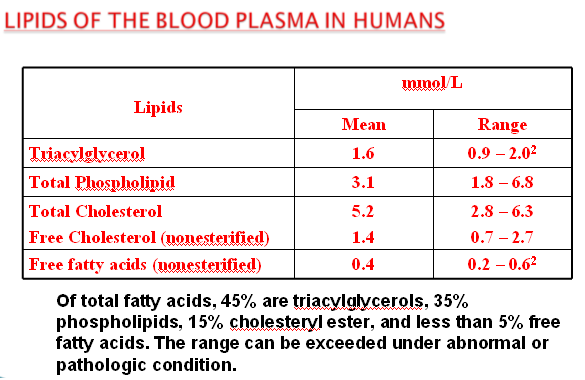
Lipid Concentrations in the Blood
TOTAL LIPIDS 360-820 mg/dL
TRIGLYCERIDES (TGs) 51-322 mg/dL
PHOSPHOLIPIDS (PL) 125-275 mg/dL
CHOLESTEROL : TOTAL 150-320 mg/dL
HDL-CHOLESTEROL 30-84 mg/dL
LDL-CHOLESTEROL 97-200 mg/dL
FREE FATTY ACIDS 6-16 mg/dL
 howMed Know Yourself
howMed Know Yourself