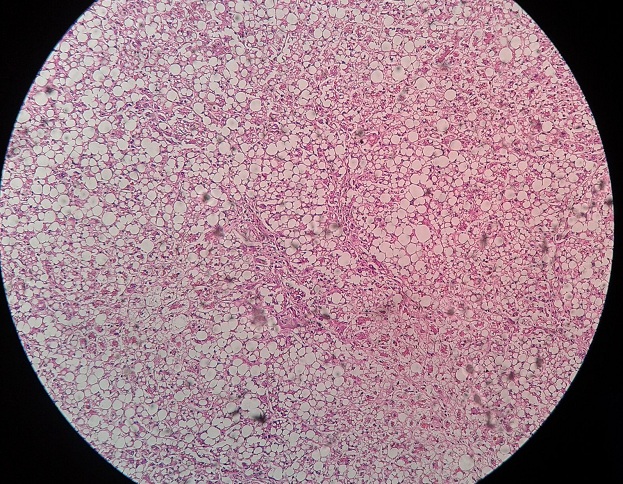
Cell death is the ultimate result of irreversible injury. It may be:
a. Physiological –e.g. during embryogenesis
b. Therapeutic –e.g. cancer radiotherapy/chemotherapy
Cell death occurs by:
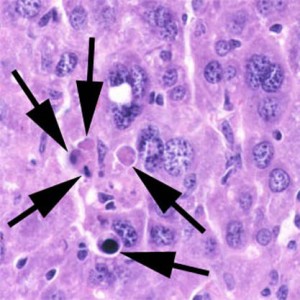
Apoptosis:
Normal programmed cell death without inflammation, e.g. during embryogenesis.
Necrosis:
Premature cell death accompanied with inflammation during evolution of disease.
Autolysis is dissolution of dead cells by own enzymes.
Cell death is recognized by:
Ultrastructural Changes
• Margination or progressive loss of nuclear chromatin
• Focal rupture of the nuclear membrane
• Breakdown of the plasma membrane.
• Development of flocculent densities in mitochondria.
Changes in the Nucleus
• Pyknosis: condensation of chromatin of chromatin and shrinkage of the nucleus.
• Karyorrhexis: fragmentation of the nucleus.
• Karyolysis: dissolution of the nucleus.
Changes in Cytoplasm Staining
• Positive staining with vital dyes such as Trepan blue which reflects abnormal membrane permeability.
• Opacification: denaturation of proteins lead to aggregation with resultant opacification of the cytoplasm.
• Eosinophilia: exposure of basic amino groups results in increased affinity for acidic dyes such as eosin.
Biochemical changes
• Release of K+ by dead cells.
• Release of enzymes into the blood. e. g. increased plasma levels of creatine kinases, lactic dehydrogenase and aspartate aminotransferase.
• Release of protein or protein breakdown products into the blood.
Postmortem change:
Degeneration autolysis of normal tissues occurring in dead body, generally distinguished from necrosis by being diffuse and not associated with inflammatory response.
Autolysis:
Digestion of cell by enzymes released from lysosome; occurs after cell dies.
After cell death
Leakage of enzymes & protein into extracellular fluid occurs, which is useful in diagnosis:
– CK-NAC, troponin in MI
– ALT in hepatitis
– ALK PO4ase in biliary obstruction
Dead cells form myelin figures with accumulation of free fatty acids leading to calcifications.
 howMed Know Yourself
howMed Know Yourself