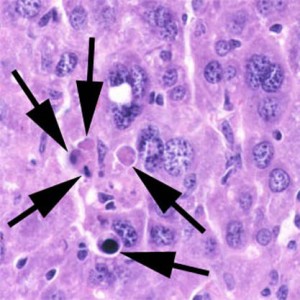
Apoptosis is the programmed cell death or suicide in which the cell membrane remains intact. No inflammatory reaction takes place.
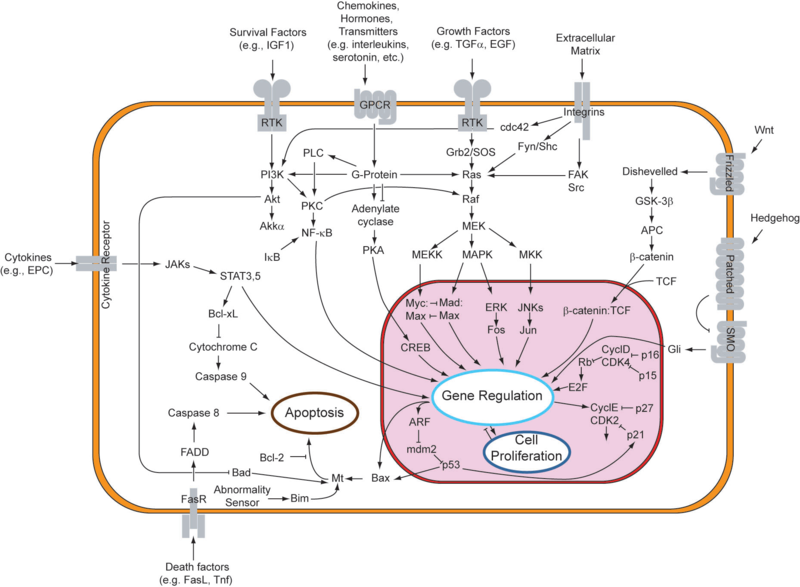
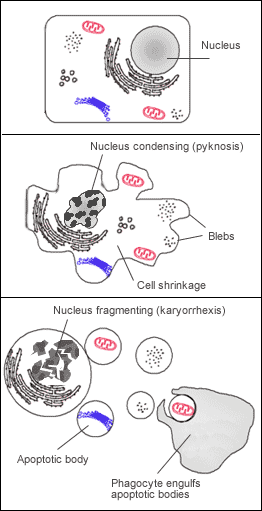
Apoptosis requires cellular signal i.e. protein cleavage within cell, causing cellular death. It is programmed and energy dependent process designed to switch off and eliminate cells. Cell shrinkage takes place, accompanied by chromatin condensation, formation of cytoplasmic blebs & apoptotic bodies and ultimately phagocytosis of apoptotic bodies.
Pathways of Apoptosis
1. Intrinsic mitochondrial pathway
As a result of increased permeability of mitochondrial membrane, cytochrome c & apoptosis inducing factor activate caspases, leading to cell death.
2. Extrinsic death receptor pathway
a. FAS & TNF1 receptor families with death domain
b. CTL CD8+
Intrinsic mitochondrial pathway
Examples of withdrawal of positive signal:
– GF for neuron
– IL-2 for lymphocytes
Examples of receipt of negative signals:
– increased intracellular fluid oxidants
– UV light, X-rays, chemotherapeutic agents
– Misfolded proteins
– TNF-α, TNF-β (lymphokines)
– Fas-ligand (Fas-L)
• Normal Bcl-2 on outer mitochondrial membrane inhibits apoptosis.
• In damaged cell, Bax protein inhibits mitochondrial Bcl-2, and punches hole in mitochondrial membrane, causing release of cytochrome-c into cytoplasm.
• Cytochrome-c binds Apaf-1 (apoptotic protease activating factor-1)
• This activates caspases 9→3 →7
Apoptosis Inducing Factor (AIF)
• Neuron does not use caspase
• AIF is located in intermembrane space of mitochondria.
• Released from mitochondria.
• AIF migrates to nucleus.
• Binds to DNA.
• Triggers destruction of DNA
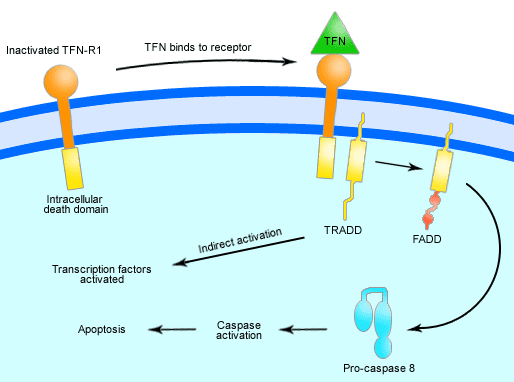
Triggering apoptosis -Extrinsic death receptor (Receptor-Ligand interaction)
a. FasL ,TNF receptor activate caspase 8, which activates executioner caspases.
b. CD8+CTL mediated lysis of their target cells.
– Directly activates executioner caspases leading to apoptosis.
– Does not use caspases cascade.
Types of Apoptosis
Physiologic apoptosis
Common examples include:
• During growth development & embryogenesis.
• Homeostatic mechanism maintains cell population
• In aging
• Shedding of menstrual endometrium
• Involution of breast after weaning
Pathologic apoptosis
Common examples include:
• Prostatic atrophy after castration.
• Death of inflammatory cells after inflammation.
• When cells are damaged by disease or injurious agents.
• DNA damage by radiation, chemotherapy, or cytotoxic drugs.
• Viral hepatitis
• Neoplasia, tumor which regress or involute
• Deletion of autoreactive T cells in thymus.
• Transplant rejection
In apoptosis, the initial ultrastructural changes consist of nuclear chromatin condensation and fragmentation, followed by cytoplasmic budding and phagocytosis of the extruded apoptotic bodies. Signs of cytoplasmic blebs, and digestion and leakage of cellular components are also visible.
Apoptosis & cancer
• HPV produces protein E6 which binds & inactivates apoptosis promoter p53.
• EBV produces protein similar to Bcl-2 & increases own Bcl-2 making infected cells more resistant to apoptosis.
• B-cell lymphoma increases levels of Bcl-2
• Melanoma inhibit expression of Apaf-1
• Colon & lung cancer cells produce blocking protein for Fas receptor, cytotoxic T lymphocytes cannot kill these tumor cells.
• Some cancer cell produce FasL which kill CTL directed against these tumor cells.
Apoptosis & Autoimmune system
Mutation is Fas , FasL or caspases result in auto reactive lymphocytes producing:
a. Autoimmune Hemolytic anemia
b. Thrombocytopenia.
Apoptosis & AIDS
Normal CD4 count is 1000/µml of blood. In AIDS it decreases to <200/µml of blood. < 1/100,000 of CD4 cells are infected by HIV. Nef HIV gene in infected cell causes high level of FasL on its surface:
– While preventing interaction of its own Fas receptor with FasL from self-destruction.
– After contact of infected CD4+ T cell with uninfected CD4 +T cell, the uninfected T-cells are killed by apoptosis.
 howMed Know Yourself
howMed Know Yourself