Alkylating agents are the broad spectrum agennts.
Chemistry
- Different reactive moiety: Bis (chloroethyl) amine / ethyleneimine / nitrosourea, responsible for different actions
- Either two or more of these are present known as Bi- / Poly-functional
Mechanism of action
- These molecules undergo intracellular circulization forming Etyleneimonium ion reacting with cellular components, further change into carbonium ion, alkylating cellular components
- In cell, different groups are present to which alkylating agents bind mainly Imidazole / amino / OH / COOH / SH / PO4 groups
- Nitrosourea – carbamoylation of lysine
- DNA – N7 G / N1,N3 A / N3 C / O6 G
- Interfere DNA synthesis, replication, transcription, alkylate one base causing joining of two bases.
- DNA cross-linking, breaks
- Interfere RNA, protein synthesis
Pharmacokinetics
- Given Oral / IV, metabolized by microsomal enzymes, mainly conjugated and excreted out.
Resistance
Different mechanisms:
1. Decreased permeation into cells
2. Increased DNA repair by cells
3. Increased production of glutathione (GL- S transferase) leading to glutathione conjugation, making alkylating agent ineffective.
Toxicity
Same as in general toxicity with some additional effects
Uses
- Blood,
- Bone marrow
- Lymphomas,
- Breast,
- Ovarian,
- Gastero-esophageal,
- Colorectal,
- Lung,
- Bladder,
- Head & neck,
- Melanoma,
- Soft tissue sarcoma,
- Neuroblastoma
Cyclophosphamide
Prodrug, commonly used
Pharmacokinetics – given orally or I/V
- metabolism occurs inliver
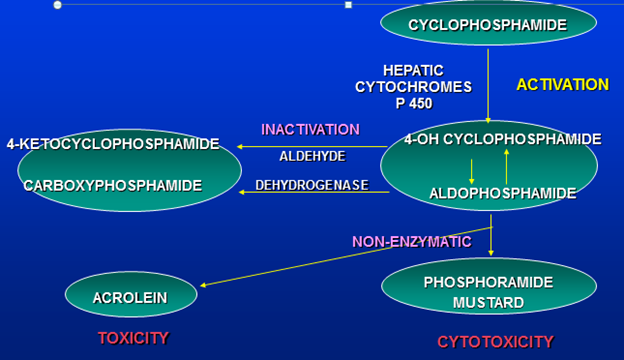
Metabolism Of Cyclophosphamide
Acrolien has side effect of hemorrhagic cystitis. Liver is protected by inactivation into 4 ketocyclophosphamide and carboxyphosphamide.
Resistance
Same as other alkylating agents
Toxicity – general
Hemorrhagic cystitis –specific effect
Provide sufficient hydration.
Mesna given –organosulphur compound which detoxifies metabolites in bladder and prevents.
Uses
- Breast,
- Ovarian,
- CLL,
- Non-hodgkin’s lymphoma,
- Wilms’tumor
Busulphan
- Used in CML
Nitrosoureas
- Carmustine,
- Lomustine,
- Streptozocin
Mechanism of Action
Alkylation (same as other alkylating agents)
- Carbamoylation of lysine
- No cross-resistance
Pharmacokinetics
–given orally or I/V
-non-enzymatic decomposition
-highly lipid soluble
Toxicity
STREPTOZOCIN has minimal bone marrow toxicity
Uses
- Insulinoma,
- Brain,
- Lymphomas
Platinum Analogs
Cisplatin - Inorganic metal complex
Mechanism of Action - same as alkylating agents
Toxicity – general
- Nephrotoxicity – hydration by oral I/V fluids
- Neuropathy
- Ototoxicity
Uses
a. Solid tumors
b. lung / esophageal / gastric / head & neck / testicular / ovarian / bladder
Carboplatin -Second generation
-Less toxic
-May cause myelosuppession
Oxaliplatin -Third generation
-for those resistant to 1st and 2nd generation
-Used in colorectal cancer (FOLFOX)
-Neurotoxicity (acute triggered by exposure to cold)
Chronic adverse effects–dose dependent)
FOLFOX – 5-FU + Oxaliplatin + Leucovorin
Continue Reading
Cancer Chemotherapy -An Introduction
Methotrexate, 5-Fluorouracil, Purine Antagonists and Antibiotics Used in Cancer Chemotherapy
 howMed Know Yourself
howMed Know Yourself