“Enzymatic modification of the drug occurring within the living organisms or chemical transformation a drug goes through in living organisms or biological fluids is known as biotransformation”.
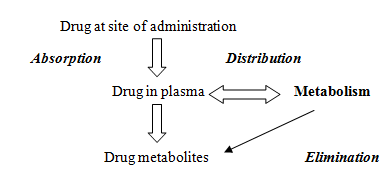
Biotransformation occurs in those foreign agents to whom the body is exposed, which may be for therapeutic, intentional, unintentional or recreational purposes. Once these agents gain entry, they undergo chemical reactions or remain inert. Whenever these drug molecules are administered, pharmacokinetic process occurs, first absorption sets into play, then distribution, metabolism and finally elimination takes place.
Biotransformation occurs only for the agents at physiological pH, having low molecular weight and less complex. Highly lipophilic, non-ionized, high molecular weight, or agents complexed with tissue or plasma proteins are not biotransformed easily.
Biotransformation is a process specifically to make agents more polar and excretable. If biotransformation does not occur, drugs may have longer duration of action, and undesired effects are observed along with desired ones. Biotransformation, in fact, is the inactivation of pharmacological action of drugs.
Biotransformation may convert drugs to active metabolites or inactive sometimes toxic metabolized products.
Drug Biotransformation Sites:
Liver is the main organ. Others include GIT, lungs, kidneys, skin, adrenals and blood (plasma).
Some of the drugs biotransformed at these sites include:
a. Liver:
Meperidine, pentazocine, morphine, nitroglycerine, lidocaine, propanolol, paracetamol, prazosin.
b. GIT:
Insulin, catecholamines, clonazepam, chlorpromazine, tyramine, salbutamol
c. Lung:
Prostanoids
d. Plasma:
Suxamethonium
Types of Biotransformation:
1. Enzymatic
a. Microsomal
b. Non-microsomal
2. Non-enzymatic (Hofmann Elimination)
Non Enzymatic Elimination:
Spontaneous, non-catalyzed and non-enzymatic type of biotransformation for highly active, unstable compounds taking place at physiological pH. Very few drugs undergo non-enzymatic elimination. Some of these include:
- Mustin HCl converted into Ethyleneimonium
- Atracurium converted into Laudanosine and Quartenary acid
- Hexamine converted into Formaldehyde
- Chlorazepate converted into Desmethyl diazepam
Enzymatic Elimination:
Biotransformation taking place due to different enzymes present in the body/cells is known as enzymatic elimination.
Non-Microsomal Biotransformation:
The type of biotransformation in which the enzymes taking part are soluble and present within the mitochondria. Examples include:
- Xanthine oxidase converting hypoxanthine into xanthine.
- Cytotoxic agent 6-mercaptocurine
- Monoamine oxidase involved in non-microsomal metabolism of catecholamines and noradrenaline.
- Alcohol dehydrogenase responsible for metabolism of ethanol into acetaldehyde
- Tyrosine hydrolases enzymes
Microsomal Biotransformation:
Enzymes responsible are present within the lipophilic membranes of endoplasmic reticulum. After isolation and putting through homogenization and fractionation, small vesicles are obtained, known as microsomes. They possess all functional, morphological properties of endoplasmic reticulum i.e. smooth and rough. Smooth ER is concerned with biotransformation and contains enzyme components while the rough ER is mainly concerned with protein synthesis.
Enzymes isolated from ER possess enzymatic activity termed as microsomal mixed function oxidase system.
Components:
- Cytochrome P450 (ferric, ferrous forms)
- NADPH (flavoprotein)
- Molecular oxygen
- Membrane lipids
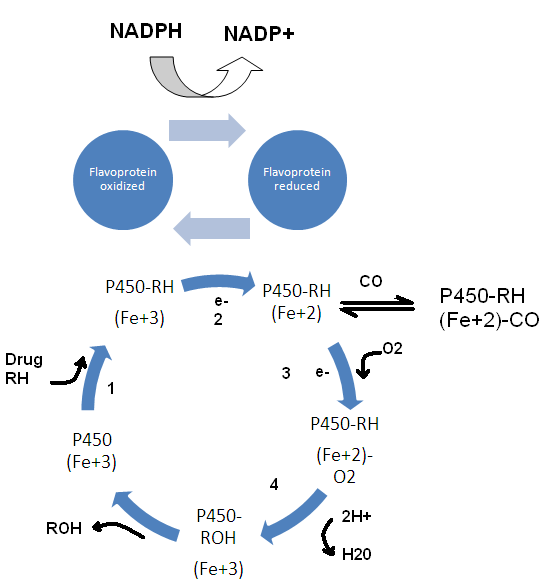
Cytochrome P450:
Cytochromes are the heme proteins, present abundantly within the living kingdom. They have about thousand known kinds. Only 50 of these heme proteins are found within the humans, which are divided into 17 families and sub-families. Name is derived because it is a heme protein (abbreviation cyp) and 450 because it reacts with carbon monoxide and during the reaction absorbs light at 450 nm.
NADPH
NADPH is a flavoprotein, less abundant than cyp 450.
For every 10 molecules of cytochrome P450, only one NADPH cytochrome reductase is present.
Cytochrome P450 cycle, RH is parent drug, ROH is oxidized metabolite, e- is electron.
Biochemical Reactions:
- Phase I reactions
- Phase II reactions
Phase I reactions:
Phase I reactions are non-synthetic catabolic type of chemical reactions occurring mainly within the ER. They are the reactions in which the parent drug is converted into more soluble excretable agents by introduction or unmasking of functional component.
Example includes phenobarbitone, aromatic hydroxylation of which abolishes its hypnotic activity. Similarly, metabolism of azathioprine produces 6-mercaptopurine.
Drug products, which are water soluble are excreted by the kidneys. Sometimes this is not true and phase I compounds do not result in true inactivation and may act as functional components of phase II reactions.
Phase I reactions include:
Oxidation
Reduction
Hydrolysis
Oxidation:
Hydroxylation
1. Hydroxylation of aromatic ring
e.g. phenobarbitone is converted into p-hydroxy phenobarbitone
2. Aliphatic hydroxylation
e.g. Meprobamate is converted into hydroxymeprobamate
Dealkylation
Conversion of mephobarbitone into phenobarbitone
O-Dealkylation
Conversion of codeine into morphine.
N-oxidation:
Conversion of aniline into nitrobenzene
Sulfoxidation
Conversion of chlorpromazine into chlorpromazine sulfoxide
Deamination
Conversion of amphetamine into phenylactate.
Desulfuration
Conversion of parathion into paraoxon
Reduction
Chloramphenicol, dantrolene, clonazepam
Hydrolysis
Esters: procaine, suxamethonium and aspirin
Amides: procainamide, lidocaine
Consequences of Phase I reactions:
1. Active drug may be converted into inactive metabolite. Active parent drug inactivation may terminate biological activity.
2. Active drug may be converted into active metabolite. E.g. morphine is converted into more active metabolite.
3. Prodrug may be converted into active metabolite
4. Active drug may be converted into toxic metabolite
e.g. halothane used in general anesthesia, is converted into trifluoroacetylated compound or trifluoroacetic acid, leading to hepatic toxicity.
5. Biotransformation of xenobiotics to mutagenic or carcinogenic agents.
6. Conversion of xenobiotics into harmless compound.
Phase II reactions:
Phase I metabolites if not readily excretable or contain component ends, combine with endogenous substrates like glucuronic acid, sulphuric acid, amino acid or acetic acid, to undergo conjugation reactions, yielding more excretable drug conjugates which are excreted by the kidneys. Phase II reactions are said to by synthetic, occurring within cytosol, while some occur within mitochondria.
Phase II reactions lead to :
- Usually inactivation of drug
- Production of water soluble metabolites, which is the main aim of biotransformation.
- Usually detoxification reaction for xenobiotics (as some substances might be carcinogenic)
- There are some important exception, toxic metabolites may be produced by their activation, e.g. methanol is converted into formaldehyde, which is toxic.
- Certain conjugation reactions may lead to the formation of reactive species responsible for the hepatotoxicity of drugs.
- Products produced might be more potent e.g. M-6-P.
Reactions are mainly conjugate reactions. Normally phase I reactions are followed by phase II reactions but in case of isoniazid (antituberculous drug), phase II reactions occur before phase I reactions because N-acetyl moiety acts as an endogenous substrate for the conjugation reaction. This is followed by phase I hydrolysis to isonicotinic acid.
Effects of Phase II reactions on drugs
- Lipid solubility is totally converted into solubility.
- Drugs are generally inactivated
- Sometimes drugs are activated e.g. minoxidil is activated to minoxidil-o-sulphate (a vasodilator). Morphine is activated to morphine-6-glucuronide.
Phase III reactions:
Some reactions coupled with excretory pumps in the cell membranes are known as phase III reactions. Drugs undergoing these reactions, is still the subject of extensive research even today.
 howMed Know Yourself
howMed Know Yourself