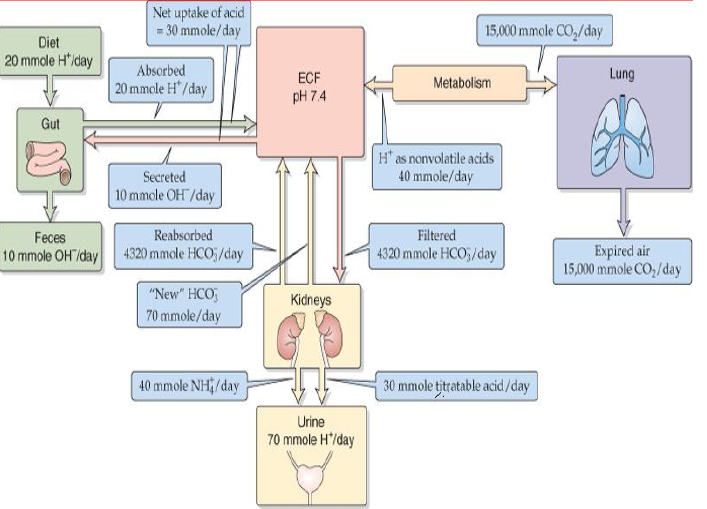
There is precise regulation or maintenance of ‘free H+ ions’ in body fluids.
Balance is Achieved by Three Defense Mechanisms:-
• First defense: Chemical buffering
• 2nd defense: Respiratory (alteration in arterial CO2)
• 3rd defense: Renal (alteration in HCO-3 excretion)
Acid Base Regulation/Balance
1. Chemical Buffer system:
– Responds within seconds
– Does not eliminate or add H+ from body
– Operates by binding or to tied up H+ till balance is reestablished.
a. In ECF:
– Mainly HCO-3/CO2 Buffer system
– Plasma Proteins
– HPO–4/H2PO-4 Buffer system
b. In ICF:
– Proteins Mainly e.g.: Hb in RBCs
– HPO–4/H2PO-4 Buffer system
Routes of excretion of acids; lungs & kidneys
2. Respiratory Mechanisms:
– Responds within minutes
– Takes 6-12 hours to be fully effective
– Operates by excreting CO2 or (adding H2CO3/HCO-3)
3. Renal Mechanisms:
• Responds slowly (effectively in 3-5 days)
• Eliminates excess Acids or Base from body
• The most powerful mechanism
e.g. i. HCO-3/CO2 Buffer system
ii. NH3/NH+4 Buffer system
iii. HPO–4/H2PO-4 Buffer system
Chemical Buffer System
• Consists of a ‘pair of substances’ present in a mixture of a solution that ‘minimizes pH changes’ when an ‘acid or base’ is ‘added or removed’ from the solution.
• Consists of;
1. Carbonic Acid – Bicarbonate Buffer System
2. Phosphate Buffer system
3. Protein Buffer system
Chemical Buffer System of ECF
1. Bicarbonate Buffer System: H2CO3/NaHCO3
consists of H2CO3 (weak Acid) + NaHCO3 (Bicarbonate salt)
– CO2 + H2O ↔H2CO3 ↔ H+ + HCO-3
– NaHCO3 ↔ Na+ + HCO-3 → H2CO3 → CO2 + H2O
Bicarbonate buffer system is quantitatively the most powerful ECF buffer system
Its two components HCO-3 & CO2 are precisely regulated by kidneys & lungs.
2. Phosphate Buffer System:
– Not of major importance in ECF
– Only 8% of the conc. of HCO-3 Buffer system
– Comprised of HPO–4/H2PO-4
– Plays major role in ICF & in Renal tubules
3. Proteins: (ICF proteins, Hb, Plasma proteins)
– Excellent buffers as proteins contain both Acidic & Basic groups.
– More important in ICF H2CO3 ← H2O + CO2
HCO-3 + H+ + HbO2 ↔ H.Hb + O2
– In RBCs, Hb is important
– 60-70% of total chemical buffering of body fluids inside the
cells & in ICF is by proteins.
– Hb buffers H+ ions generated by H2CO3
– Proteins are the most abundant buffers in cells & in blood
– Histidine and Cysteine are the two A. Acids that contribute
most of the buffering capacity of proteins
Respiratory Mechanisms in Regulation of Acid-Base
• Second line of defense against acid base disturbances
• Operates through regulation of ECF CO2 concentration by lungs
• Effectiveness between 50-75% [feedback gain is 1-3 i.e. fall in pH
from 7.4 to 7.0 is returned by Resp System to 7.2 to 7.3 within 3-12
minutes]
CO2 :
– ↑PCO2 → ↑Ventilation →Eliminates CO2 → Reduces [H+] & ↑pH
– ↓PCO2 → ↓Ventilation → ↑CO2 → ↑ [H+] & ↓ pH
– Doubling the ventilation → ↑pH
(about 0.23 units) i.e 7.4 → 7.63
– ¼ of normal ventilation → ↓ pH
(about 0.45 units) i.e 7.4 → 6.95
[H+] :
– ↑[H+] → ↑Alveolar Ventilation →↓CO2
– ↓pH (from 7.4-7.0) → ↑Alv. Vent by 4 times normal.
– ↑pH → ↓Alv. Vent.
– Change in Vent. Rate per unit change in pH is much greater at low pH as compared with that of increased levels of pH
Reason: ↑pH→↓PO2 →Stimulate Vent. Therefore, respiratory compensation is not effective!
– Respiratory Mechanism has effectiveness between 50-75% & is 1-2 times as great as the buffering power of all other chemical buffers in ECF.
Renal Mechanisms in Regulation of Acid-Base
Kidneys operate through;
i. Active secretion of H+ ions
ii. H+ ion buffering within tubular lumen:
a. buffering with HCO-3;
Result in reabsorption of filtered HCO-3 ions
b. buffering with HPO–4 orNH3 ;
Result in H+ ion excretion & generation of new HCO-3 ions
Outcome: by excreting acidic or basic urine
• Process is achieved by three buffer systems;
1. Carbonic Acid buffer system
2. Ammonia buffer system
3. Phosphate buffer system
• Kidney’s acid-base regulatory potency is that it has ability to return the pH
almost exactly to normal.
Carbonic Acid Buffer System
Daily Reabsorption of HCO-3:
85% HCO-3 reabsorption (H+ Secretion) occurs in PCT
10% HCO-3 reabsorption (H+ secretion) occurs in thick ascending LOH
4.9% (approx 5%) reabsorption (H+ secretion) occurs in DCT & CT.
For each HCO3 reabsorbed, there must be one H+ ion secreted.
• Secretion of H+ in PCT: by two mechanisms;
• Via Na+-H+ antiporter (major route)
• Via H+-ATPase (proton pump)
The net effect is the reabsorption of one HCO-3 & one Na+ for secretion of one H+
However, this secreted H+ is consumed in reaction with filtered HCO-3
Phosphate Buffer System in Renal Tubules
• Consists of HPO–4 & H2PO-4 (Both are poorly reabsorbed in renal tubules, get concentrated by reabsorption of H2O)
• Operates when secreted [H+] is in excess than filtered [HCO-3]
• Under normal conditions 30-40 mEq/day filtered phosphate is available for buffering H+.
• Much of the buffering of excess H+ ions in tubular fluid is carried out by ‘Ammonia Buffer System’
When there is excess H+ ions in ECF:
• Kidneys takle it by;
1. Reabsorption of all filtered HCO-3
2. Generation of new HCO-3 (to replenish decreased level of HCO-3 in ECF)
Ammonia Buffer System
• 2nd Special Buffer System
• Consists of NH3 & NH+4
• More important ‘quantitatively’ than phosphate buffer system.
• NH4 ions are synthesized from glutamine in PCT, secreted into tubular fluid
by Na+-H+ exchange (counter transport) mechanism i.e. NH+4 is secreted in place of H+.
Ammonia Buffer System in Renal Tubules
Fate of NH+4 : (After its secretion into lumen of PCT)
• A portion of NH+4 is excreted directly in urine.
• Remainder NH+4 is reabsorbed in thick ascending limb of LOH; enters medullary interstitium; then secreted from medullary interstitial fluid into collecting ducts for final excretion.
• Through α-intercalated cells (H+-ATPase) under the influence of aldosterone.
• Under normal conditions: Ammonia buffer system accounts for
50% elimination of H+ ions & 50% new HCO-3 are generated by kidneys.
• In Chronic Acidosis: Rate of NH+4 excretion increases as much
as 500 mEq/day by enhancing glutamine metabolism in kidneys.
Disturbances of Acid Base Balance
• Primary change in ECF [HCO-3] = Metabolic
• Primary change in ECF PCO2 = Respiratory
Acidosis : ↓ pH less than 7.35
– Primary ↓[HCO-3] = Metabolic (↓ HCO-3, ↓ PCO2)
– Primary ↑PCO2 = Respiratory (↑ HCO-3, ↑ PCO2)
Alkalosis: ↑ pH more than 7.45
– Primary ↑[HCO-3] = Metabolic (↑ HCO-3, ↑ PCO2)
– Primary ↓ PCO2 = Respiratory (↓ HCO-3, ↓ PCO2)
Acid Base Imbalance
Can arise due to:
1. Respiratory Dysfunction
2. Metabolic Dysfunction
Changes in [H+] are reflected by changes in Ratio of [HCO-3] to [CO2]
• Normal Ratio = 20/1 {[HCO-3] = 24 mM/L, [CO2]= 1.2 ml/L}
Ratio < 20/1 = Acidosis
Ratio > 20/1 = Alkalosis
• Change in blood pH (Acidosis or Alkalosis) are counteracted by physiological
responses of Buffer Systems (Lungs + kidneys) called compensation.
• Altered blood pH of ‘metabolic origin’ is helped by ‘respiratory
compensation’ (Change in PCO2)
• Altered blood pH of ‘respiratory origin’ is helped by ‘renal
compensation’ (change in [HCO-3])
Respiratory Acidosis:
• ↓ pH < 7.35 (Uncompensated)
• ↑ PCO2 > 45 mmHg
Causes:
Hypoventilation (e.g. Emphysema)
Pulmonary Edema
Trauma to Resp center
Airways obstruction, pneumonia, emphysema,
↓surface area of pulmonary membrane
Dysfunction of Resp Muscles
Compensatory Mechanisms:
↑ Renal excretion of H+
↑ Reabsorption of HCO-3
• If compensated:
pH within normal range & PCO2 = High
Respiratory Alkalosis:
• ↑ pH > 7.45
• ↓ PCO2 < 35 mmHg
Causes:
• Hyperventilation (may be hypoxic)
• Pulmonary disease, Anxiety
• CVA, Aspirin overdose
Compensatory Mechanisms:
• ↓ Renal excretion of H+
• ↓ Reabsorption of HCO-3
If compensated;
• pH within normal Range, PCO2 = low
Metabolic Acidosis:
• ↓ pH < 7.35 (uncompensated)
• ↓ HCO-3 < 22 mEq/L
Causes:
• Loss of HCO-3 due to severe diarrhea (most common)
• Accumulation of acid e.g. Ketoacidosis
• Renal dysfunction
Compensatory Mechanism:
• Respiratory : Hyperventilation (↑CO2 loss)
If compensated;
• pH within normal range, HCO-3 = low
Metabolic Alkalosis:
• ↑ pH > 7.45 (uncompensated)
• ↑ HCO-3 > 26 mEq/L
• Causes:
• Loss of acid due to vomiting*
• Gastric suction*
• Use of diuretics
• Excessive intake of alkaline drugs (antacids)
Compensatory Mechanisms:
• Respiratory; Hypoventilation (slow loss of CO2)
If compensated;
• pH = within normal range, HCO-3 = High.
Contraction Alkalosis
↓ECF →↓Bl Vol →↓Renal perfusion→↑Renin, AgII & Aldo→↑Na-H+ exchange &↑HCO3 reabsorption in PCT, H+ excretion by Aldo
* “Contraction alkalosis”
Anion Gap
• Anion gap derives from the principle of ‘electroneutrality’.
• Routinely some cations & anions are measured and others are not (Na+,HCO-3, Cl-)
• When conc. of Na+ is compared to sum of HCO-3 & Cl- , there is an anion gap i.e. conc. of Na+ is greater than sum of HCO-3 & Cl-.
• To keep electroneutrality , plasma must contain unmeasured anions to make up difference.
• Plasma anion gap = 8-16 mEq/L.
=[Na+] – ([HCO-3] + [Cl-])
= 144 – 24 – 108
= 12 mEq/L (Normal)
• Plasma anion gap is primarily useful in differential diagnosis of metabolic acidosis.
• Increased anion gap: e.g. metabolic acidosis, starvation, CRF
An accumulation of an organic anion; e.g. ketoacid, lactate, formate, salicylate. (Decrease in HCO-3 conc. is offset by an increase in conc. of an unmeasured organic anion; albumin, phosphate, sulfate & other anions).
[Unmeasured cations include; Ca++, Mg++ & K+]
• Normal anion gap:
No accumulation of an organic anion, but decrease in HCO-3 conc. is offset by an increase in conc. of Cl-
‘Hyperchloremic metabolic acidosis’ with normal anion gap
(e.g. diarrhea, renal tubular acidosis)—- ‘nonanion gap’.
 howMed Know Yourself
howMed Know Yourself