What are Enzymes?
Biological catalysts which speed up the rate of reaction without becoming part of the reaction
Enzymes increases the rate of reaction by lowering the activation energy barrier, thus allowing reactions to proceed without an input of energy
Enzyme Nomenclature
Recommended Name:
Suffix “ase” attached to the susbtrate of the reaction
For example; Glucosidase, Urease
Systematic Name
IUBMB divided enzymes into six classes
The suffix “ase” is attached to describe complete chemical reaction and substrate name
For example; Pyruvate decarboylase
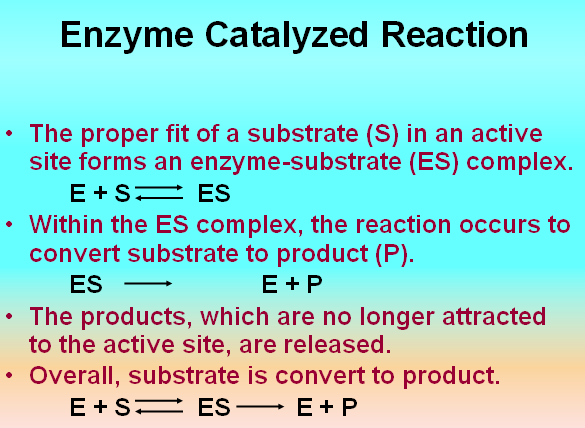
How Enzyme Works
• Active site – a region of an enzyme comprised of different amino acids where catalysis occurs
• Substrate – the molecule being utilized and/or modified by a particular enzyme at its active site
• Co-factor – organic or inorganic molecules that are required by enzymes for activity.
Enzymes convert substrates into products
• What is a substrate?
– A substrate is the compound that is converted into the product in an enzyme catalyzed reaction.
Physical Nature
• Apoenzyme = the protein part of an enzyme without coenzymes or prosthetic groups that are required for the enzyme to have activity.
• Coenzyme = small organic molecules which is dialyzable, thermostable and loosely attached to the protein part.
• Prosthetic group = an organic substance which is dialyzable and thermostable which is firmly attached to the protein or apoenzyme portion.
• Cofactors= Inorganic molecules
Cofactors
• Non-protein molecules that help enzymes function.
• Bind to active site to enhance enzymatic reactions.
• Cofactors may be inorganic metals such as zinc, iron, or copper.
COENZYMES
• Heat stable, low mol wt organic compounds, non-covalently linked with enzymes, can be separated.
APO + CO = Holoenzyme
Act as intermediate or ultimate acceptor in group transfer
Important Coenzymes
• NAD+
• NADP+
• FAD
• Coenzyme A
REDUCTION OF FAD OR FMN TO FADH2 OR FMNH2
FMN is co enzyme for cytochrome C oxidase, L.Amino acid dehydrogenase
FAD is co-enzyme for xanthene oxidase acyl-CoA dehydrogenase
Ascorbic acid (Vitamin C)
– Required for hydroxylation of proline into hydroxyproline for synthesis of collagen
– Bile acid formation
– Maintain metalic co-factors like Cu+ in Monooxygenases and Fe in dioxygenases
– Conversion of cholesterol into steroid hormone in adrenal cortex
– Absorption of iron
– Acts as antioxidant in GIT by preventing formation of nitrosamines during digestion
• Folic acid
– Acts as single carbon carrier for synthesis of various compounds like pyrimidines and purines
• Vitamin B12
– Acts as co-enzyme in groups rearrangements in isomerases e.g. conversion of methyl malonyl CoA into succinyl-CoA by enzyme methylmalonyl-CoA mutase
– Converts homocysteins into methionine
– Act as maturation factor for RBCs
CLASSIFICATION of ENZYMES
1) Oxidoreductases
Catalyze the transfer of hydrogen atoms and electrons
Example – Lactate Dehydrogenase
Subgroup of Oxidoreductase
A. Oxidases cause Oxidation
Ascorbic acid oxidase, Xanthine oxidase
B. Dehydrogenases Removes 2H to form double bond
Lactate Dehydrogenase, Glucose-6-Phospahte Dehydrogenase
C. Hydroperoxidases Require H2O2 as hydrogen acceptors
Peroxidase, Catalase
D. Oxygenases Addition of oxygen to the substrate
Phenylalanine hydroxylase, Tryptophan dioxigenase
2)Transferases
Transfer of functional group
Sub-Classes of Transferases
1. Transaminases – of amino group
Aspartate transaminase (AST)
2. Transmethylases – of a methyl group
Catechol O methyltransferases
3. Transacetylases – of an acetyl group
choline acetyltransferas
4. Transglycosidases – transfer of one glycoside to another
β-glycosidase
5. Transphosphorylases- of a phosphate group
Hexokinase, glucokinase
3)Hydrolases
cleavage of different bonds (C-C, C-O, or C-N bonds) by the addition of water (hydrolysis) maltase, pepsin, trypsin
4)Lyases
Cleavage of C-C, C-O, or C-N bonds
(addition of groups to double bonds or formation of double bonds by removal of groups) fumarase
5) Isomerases
catalyze the transfer of functional groups within the same molecule. isomerase
6) Ligases
use ATP to catalyze the formation of new covalent bonds. succinate thiokinase
MICHEALIS – MENTON EQUATION
V1 = V max [s]
Km + {S}
V1 = Measured initial velocity
V max = Maximum velocity
S = Substrate
Km = Michaelis constant
Factors Influencing Enzyme Activity
a) Enzyme concentration
b)Temperature
c)pH
d)Substrate concentration
e)Inhibitors
TYPES OF INHIBITORS
Reversible Inhibitor
Competitive
Non-Competitive
Uncompetitive
Irreversible Inhibitor
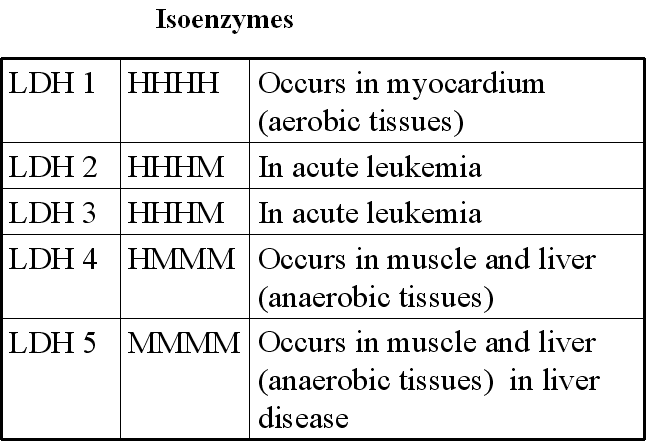
What is an isozyme?
(1) Isozymes are physically distinct forms of the same enzyme.
(2) Isozymes may differ from each other by differences in their amino acid sequences
(3) The relative abundance of different isozymes varies for different tissues.
Lactate dehydrogenase on electrophoresis gives 5 different bands and has 4 protomers
Medical Relevance
• Many diseases are caused by the absence, malfunction, or inappropriate expression of a particular enzyme—SOD
• Enzymes serve as targets for a variety of drugs
• Enzymes are sometimes administered in the treatment of disease
• The presence or absence of specific enzymes can be used to diagnose specific diseases
Clinical Use of Enzymes
• Enzyme Activity in Body Fluids Reflects Organ Status:
• Cells die and release intracellular contents; increased serum activity of an enzyme can be correlated with quantity or severity of damaged tissues (ex. creatine kinase levels following heart attack)
• Increased enzyme synthesis can be induced and release in serum correlates with degree of stimulation (ex. alkaline phosphatase activity as a liver status marker)
• Enzyme activity can be altered genetically
• A mutation in an enzyme can alter its substrate affinity, co-factor binding stability etc. which can be used as a diagnostic in comparison with normal enzyme
• Loss of enzyme presence due to genetic mutation as detected by increased enzyme substrate and/or lack of product leading to a dysfunction
Serum enzymes are commonly used in diagnostic tests for a variety of diseases
Myocardial Infarction: Lactate dehydrogenase (H4 isozyme), Aspartate aminotransferase, Creatine kinase
Viral hepatitis: Alanine aminotransferase
Acute pancreatitis: Amylase, Lipase
Liver disease: Alkaline phosphatase, Lactate dehydrogenase (M4 isozyme)
Salivary Gland Inflammation:
• In Mumps:
• The levels of a-Amylase (AMS) is significantly increased
Muscle Disorders
• Muscle dystrophy.
• Muscle trauma.
• Muscle hypoxia.
• Frequent I.M Injections.
• The plasma levels of the following enzymes increase
CK. LDH/ AST
Malignancies
a) Plasma (Acid phosphatase) ACP
levels increase in:
• Prostatic carcinoma.
• Bone metastatic carcinoma
b) Plasma levels of Alkaline
phosphatase (ALP) increase in:
• Pancreatic carcinoma.
• Bile duct carcinoma.
• Liver metastasis.
 howMed Know Yourself
howMed Know Yourself

I am extremely impressed with your writing skills and also with the layout on your blog. keep up the nice quality writing, it’s rare to see a nice blog like this one these days.. 🙂
You clearly saved me atleast 1 hour of time. I am making a project in this particular topic and your write-up has helped me through one of the topics of my project. I will browse to the other pages now.
Nice piece of data that you’ve got in this web site submit. Hope I can get some far more of this stuff on your web site. I’ll are available back.