Atrophy may be defined as the shrinkage in size of cell by loss of cell substance, resulting in decreased functional ability. It is the decrease in cell size & number.
Atrophy is a form of adaptive response. Atrophic cells may have diminished function but they are not dead. However, atrophy may progress to cellular death.
Causes of Atrophy
1. Physiologic Causes
a. Loss of hormonal stimulation
Atrophy of uterus after parturition takes place.
b. Aging
Aging process is associated with cell loss, typically seen in brain and heart.
2. Pathologic Causes
Pathologic causes may include:
a. Disuse
In broken limb immobilized in a plaster cast, muscle atrophy ensues.
b. Denervation
Damage to nerves leads to rapid atrophy of muscle. E.g. viral infection damages anterior horn cells leading to poliomyelitis.
c. Ischemia
In late adult life, the brain undergoes atrophy progressively as atherosclerosis narrows blood channels.
d. Cachexia
Protein calorie malnutrition is associated with the use of body of skeletal muscles for source of energy after other residues such as adipose stores have been depleted. This results in marked muscle wasting.
e. Loss of endocrine stimulation
E.g. loss of estrogen.
f. Pressure
Tissue compression for any length of time can cause atrophy. An enlarged bengin tumor can cause atrophy in surrounding compressed tissue, probably due to ischemic changes.
g. Malnutrition
Types of Atrophy
Atrophy may be of two types:
1. Physiologic atrophy
Common examples are:
• Atrophy of notochord & thyroglossal ducts during fetal development
• Atrophy of uterus after parturition.
• Atrophy of ductus arteriosus in infants
• Atrophy of thymus
2. Pathologic atrophy
Depending upon the underlying cause, pathologic atrophy may be localized or generalized:
a. Generalized
e.g. inadequate nutrition
b. Localized
e.g. loss of innervation, loss of endocrine stimulation or diminished blood supply.
3. Ageing –Senile atrophy
Brown Atrophy
Some cell debris, within autophagic vacuoles, may resist digestion and persist as membrane bound residual bodies, that may remain as a sarcophagus in the cytoplasm. An example of residual body is lipofuscin granules. When they are present in sufficient amounts, they impart brown color to the tissues.
Mechanism of Atrophy
Balance between protein synthesis and degradation is affected. Either there is decreased protein synthesis or increased protein degradation by multiple proteolytic systems.
a. Lysosomes contain hydrolases (cathepsin) and other enzymes which degrade endocytosed proteins.
b. Decreased protein synthesis leads to decreased metabolic activity, which in turn leads to increased protein degradation by ubiquitin-proteasome pathway. Activation of ubiquitin ligases attach peptide ubiquitin to cellular proteins & target these proteins for degradation in proteasomes. Increased autophagy results in i ncreased autophagic vacuoles, leading to increased residual bodies (lipofuscin).
c. Increased number of autophagic vacuoles into which lysosomes discharge their hydrolytic contents.
Glucocorticoids and thyroid hormones stimulate proteasome-mediated protein degradation; insulin opposes these effects. Cytokines such as tumor necrosis factor TNF are capable of increasing muscle proteolysis.
Morphological Features
Microscopy
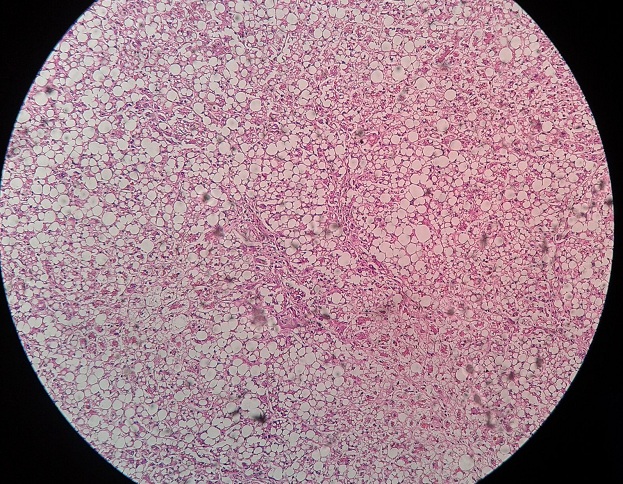
Small, shrunken cells with lipofuscin granules (golden brown wear and tear pigment derived from subcellular membrane).
Electron Microscopy
Decreased intracellular components and autophagosomes. Atrophic muscle cells contain fewer mitochondria, myofilaments and decreased amount of endoplasmic reticulum.
Want a clearer concept, see
 howMed Know Yourself
howMed Know Yourself