Chloramphenicol is a broad spectrum antibiotic and protein synthesis inhibitor.
Source
- Isolated from cultures of streptomyces in Venezuela – 1947
- Synthetically prepared – 1948
Chemistry
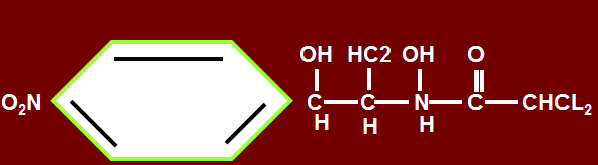
- Contains nitrobenezene moity and is a derivative of dichloracetic acid, which contributes to blood dyscrasias producing life threatening side effects. Clinically use is restricted to life threatening situations only (beta lactams, 3rd gen cephalosporins, etc. are used as uses same).
- Biologically active form is levorotatory.
Mechanism of action
- Chloramphenicol inhibits protein synthesis
- In bacteria, penetrates bacterial cells by facilitated diffusion.
- Binds reversibly to the 50 s ribosomal subunit &inhibits transpeptidation reaction
- Inhibit mitochondrial protein synthesis
- In mammalian cells, 70S mitochondrial ribosomes resemble bacterial ribosomes, which might also be inhibited
- Thus there are chances of toxicity in erythropoietin cells.
Anti microbial spectrum
Effective against gram positive and negative cocci, predominantly against anaerobes.
- Gram positive organisms
- Gram negative organisms
- Rickettsiae. (drug of choice)
- H. Influenzae,
- N. meningitidis, N gonorrhoea,
- Salmonella typhi,
- Brucella sp,
- Bordetella pertusis
Anaerobic bacteria
- Gram positive cocci and clostridium,
- Gram negative rods,
- B. Fragilis
Enterobacteriaceae
- E- coli,
- Proteus mirabilis,
- Pseudomonas,
- Pseudomalli v.
- Cholera,
- Shigella,
Pharmacokinetics
- Prodrug
- Available as such and as crystalline chloramphenicol base.
- Oral administration: chloramphenicolpalmitate. When administered orally, in intestine it is hydrolyzed by pancreatic lipases and free chloramphenicol is released. The entire chloramphenicol base gets absorbed and has good bioavailability.
- Chloramphenicol succinate, water, oil soluble preparation given orally and I/M.
- About 60% of the drug reaches CSF as compared with plasma. Higher concentration in brain is used in bacterial meningitis. It is drug of choice due to higher concentration in brain.
- When administered I/V, it is hydrolyzed by esterases in lungs, kidneys and liver. 20-30% of prodrug is excreted by kidneys, remaining is hydrolyzed by esterases. Plasma levels are less after I/V administration as compared with oral.
- Oral bioavailability is more.
- Metabolized in liver by glucuronide transferrase. 90% is conjugated, 10% is reduced to aryl amines by reduction.
- Higher concentration of drug crosses placenta. It is also secreted in aqueous humor after subconjunctival injection. Topical eye ointment for conjunctivitis and corneal ulcers are also available for better penetration into aqueous humor.
Therapeutic uses
It was evident in 1950 that drug could cause fatal blood dyscrasias.
Use of the drug is reserved for patients with serious infections such as:
- Meningitis,
- Typhus,
- Typhoid fever (14 days)(ampicillin, ciprofloxacin normally given)
- Rocky mountain spotted fever (chloromycetin)
- Oily chloramphenicol is recommended by WHO as the first line treatment of meningitis in low income countries and appears on essential drugs list. It is the cheapest treatment available for meningitis.
- Single injection first line in the treatment of Staphylococcal brain abscesses.
- Septicemia
- Acromegaly (brain infection may develop)
- May be used against enterobacteriaceae
- Precancerous polyps
There are 3 main bacterial causes of meningitis:
- Nesseria meningitides
- Streptococcus pneumonia
- Haemophilus influenzae
Resistance:
- Resistance to chloramphenicol is caused by a plasmid encoded acetyltransferase that inactivates the drug.
- Effects membrane permeability.
- Different effects in gram positive and gram negative bacteria.
Untoward effects/ adverse effects
1. Hematological:
Continuous therapy for 2 weeks or more causes two types of effects:
a. Reversible –dose related
- Anemia
- Leukopenia
- Thrombocytopenia
b. Irreversible
Idiosyncratic (genetic predisposition)- leading to pancytopenia that is aplastic anemia, which accounts for approx. 70% cases of blood dyscrasias due to chlorampehnicol
2. Hypersensitivity reactions:
- Jarisch – Herxheimer
- Reactions may occur after chloramphenicol is administered for secondary syphilis, brucellosis & thyphoid fever.
3. GIT:
a. nausea
b. vomiting
c. unpleasant taste
d. diarrhea
4. Rare toxic effects:
Appear after I/V administration.
- Blurring of vision,
- Digital paresthesias,
- Optic neuritis
- Fatal chloramphenicol toxicity may develop in neonates that is gray baby syndrome. Drug is not detoxified if administered during 1st week, glucuronyl transferrase is not active, thus the drug accumulates. Beta lactam drugs are given during first week. If needs to be given at all, give a lower dose (meningitis, encephalitis).
Drug interactions
1. As bacteriostatic, not combined with penicillin.
2. Inhibits hepatic cytochrome p 450 enzyme, thus inhibits the metabolism of drugs metabolised by this system, prolonging their half life i.e
a. warfarin,
b. phenytoin,
c. chlorpropamide
d. Antiretroviral protease inhibitors – rifabutin
Severe toxicity and death has occurred because of failure to recognize such effects.
 howMed Know Yourself
howMed Know Yourself